



Russell J. Donnelly
541-346-4226 (Tel)
541-346-5861 (Fax)
![]()
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |
![]() The
Observed Properties of Liquid Helium
The
Observed Properties of Liquid Helium
![]() at the Saturated Vapor
Pressure
at the Saturated Vapor
Pressure
![]() Russell J. Donnelly and Carlo F. Barenghi
Russell J. Donnelly and Carlo F. Barenghi
The
equilibrium and transport properties of liquid 4He are deduced from experimental
observations at the saturated vapor pressure. In each case the bibliography
lists all measurements known to us. Most of these quantities are represented
by cubic spline fits of a data base adopted from the cited literature
by subjective judgement. Quantities reported here include density, thermal
expansion coefficient and dielectric constant, superfluid and normal fluid
densities, first, second, third and fourth sound velocities, specific
heat, enthalpy, entropy, surface tension, ion mobilities, mutual friction
,viscosity and kinematic viscosity, dispersion curve, structure factor,
thermal conductivity, latent heat, saturated vapor pressure, thermal diffusivity
and Prandtl number of helium I, displacement length and vortex core parameter
in helium II. This report differs from an earlier one by J. S . Brooks
and R. J. Donnelly (J. Phys. Chem. Ref. Data 6, 51, 1977) in which equilibrium
properties of helium II were calculated from neutron scattering experiments
over a range of temperatures and pressures.
Key words:, helium I, helium II, observed equilibrium and transport properties
of liquid 4He.
Table
of Contents
Chapter |
Title |
Page |
| Introduction Acknowledgements List of Tables List of Figures |
ii v vi ix |
|
| 1 | Density, thermal expansion and dielectric constant | 1 |
| 2 | Superfluid and normal fluid densities | 11 |
| 3 | First sound velocity | 20 |
| 4 | Second sound velocity | 30 |
| 5 | Third sound velocity | 40 |
| 6 | Fourth sound velocity | 43 |
| 7 | Specific heat and enthalpy | 49 |
| 8 | Entropy | 66 |
| 9 | Surface tension | 75 |
| 10 | Ion mobilities | 83 |
| 11 | Mutual friction | 100 |
| 12 | Viscosity and kinematic viscosity | 105 |
| 13 | Dispersion curve | 114 |
| 14 | Structure factor | 120 |
| 15 | Thermal conductivity | 128 |
| 16 | Latent heat | 137 |
| 17 | Saturated vapor pressure | 145 |
| 18 | Thermal diffusivity of helium I | 151 |
| 19 | Prandtl number of helium I | 153 |
| 20 | Displacement length and vortex core parameter | 154 |
| Appendix | Programs for spline evaluation | 156 |
List of Figures
1.1 |
The recommended values of the density of liquid 4He as a function of temperature at the saturated vapor pressure................................................ |
1223 |
| 1.2 | The recommended values of the thermal expansion coefficient of liquid 4He at the saturated vapor pressure as a function of temperature.............................. | 1223 |
| 1.3 | Detail of the recommended values for the thermal expansion coefficient of liquid 4He near the lambda transition..................................................... | 1223 |
| 1.4 | The recommended values of the thermal expansion coefficient of liquid 4He at low temperatures...................................................................................... | 1223 |
| 2.1 | Recommended values of the superfluid density of helium II as a function of temperature...................................................................................................... | 1226 |
| 2.2 | Recommended values of the superfluid density of helium II near the lambda transition...................................................................................................... | 1226 |
| 2.3 | The fractional deviation of the adopted database from the recommended values for the superfluid density of helium II expressed in percent....................................... | 1226 |
| 3.1 | The recommended values for the first sound velocity of liquid 4He as a function of temperature at saturated vapor pressure............................................................ | 1228 |
| 3.2 | Detail of the recommended values for the first sound velocity of liquid 4He about the lambda transition........................................................................................ | 1228 |
| 3.3 | The fractional deviation of the adopted database from the recommended values of the velocity of first sound in liquid 4He expressed in percent................................. | 1228 |
| 3.4 | The fractional deviation of the adopted database from the recommended values of the velocity of first sound in liquid 4He expressed in percent.for ............................ | 1228 |
| 3.5 | The fractional deviation of the adopted database from the recommended values of the velocity of first sound in liquid 4He expressed in percent. for .......................... | 1230 |
| 4.1 | The recommended values for the velocity of second sound in helium II as a function of temperature at saturated vapor pressure......................................................... | 1231 |
| 4.2 | Detail of the recommended values for the second sound velocity in helium II about the lambda transition......................................................................................... | 1231 |
| 4.3 | The fractional deviation of values of the adopted data base from the recommended values for the velocity of second sound in helium II expressed in percent................ | 1231 |
| 6.1 | The recommended values for the velocity of fourth sound as a function of temperature at the saturated vapor pressure....................................................... | 1235 |
| 6.2 | The fractional deviation of values of the adopted database from the recommended values for the velocity of fourth sound in helium II expressed in percent................. | 1235 |
| 7.1 | The recommended values for the heat capacity of liquid 4He as a function of temperature at saturated vapor pressure............................................................ | 1236 |
| 7.2 | The
recommended values for the heat capacity of liquid 4He
at saturated vapor pressure near expressed as Cs vs log10
| T-T |
1236 |
| 7.3 | The fractional deviation of values of the adopted database from the recommended values for the heat capacity of liquid 4He expressed in percent | 1237 |
| 7.4 | The
fractional deviation of values of the adopted database from the recommended
values for the heat capacity of liquid 4He near the lambda transition
for T < T |
1237 |
| 7.5 | The
fractional deviation of values of the adopted database from the recommended
values for the heat capacity of liquid 4He near the lambda transition
for T > T |
1237 |
| 7.6 | Values for the enthalpy of liquid 4Hehelium at saturated vapor pressure, obtained by integration of the recommended values of the heat capacity............................ | 1237 |
| 7.7 | Fractional deviation of the spline fit of the recommended values of the enthalpy from the enthalpy integrated from recommended values of the heat capacity......... | 1237 |
| 8.1 | The recommended values of the fountain pressure entropy of liquid 4He as a function of temperature at saturated vapor pressure.......................................... | 1243 |
| 8.2 | The fractional deviation of values of the adopted database from the recommended values for the fountain pressure entropy of liquid 4He expressed in percent.......... | 1243 |
| 8.3 | The recommended values of the entropy of liquid 4He obtained by integration of the heat capacity spline....................................................................................... | 1243 |
| 8.4 | The fractional deviation of values of the adopted database from the recommended values for the entropy integrated from the heat capacity spline.............................. | 1244 |
| 9.1 | The recommended values of the surface tension of liquid 4He as a function of temperature at saturated vapor pressure............................................................ | 1245 |
| 9.2 | Detail of the surface tension about the lambda transition | 1246 |
| 9.3 | The fractional deviation of the values of the adopted database from the recommended values of the surface tension of liquid 4He expressed in percent | 1246 |
| 10.1 | Positive ion mobility as a function of temperature along the saturated vapor pressure line................................................................................................... | 1248 |
| 10.2 | Deviation
of the database from the spline fit to log10 |
1248 |
| 10.3 | The recommended values of the negative ion mobility as a function of temperature at saturated vapor pressure.............................................................................. | 1248 |
| 10.4 | The
fractional deviation of values of the adopted database from the recommended
values for the log10 |
1248 |
| 12.1 | The recommended values of the viscosity of liquid 4He as a function of temperature at saturated vapor pressure.............................................................................. | 1255 |
| 12.2 | The fractional deviation of values of the adopted database from the recommended values for the viscosity of liquid 4He expressed in percent..................................... | 1255 |
| 12.3 | The
recommended values of the kinematic viscosity of liquid 4He
v= |
1256 |
| 13.1 | Energy E in degrees Kelvin of elementary excitations in helium II as a function of wave number Q (Ref.12).................................................................................... | 1258 |
| 13.2 | Group velocity of elementary excitations in helium II as a function of wave number Q (Ref.12........................................................................................................ | 1259 |
| 13.3 | The fractional deviation of values of the adopted database from the recommended values for the dispersion curve for liquid 4He, expressed in percent....................... | 1259 |
| 14.1 | The recommended values for the structure factor of liquid 4He as a function of wave number............................................................................................................ | 1260 |
| 14.2 | The fractional deviation of values of the adopted database from the recommended values for the structure factor expressed in percent............................................. | 1262 |
| 15.1 | The recommended values for the thermal conductivity of liquid 4He as a function of temperature at the saturated vapor pressure...................................................... | 1262 |
| 15.2 | Log
plot of k vs | T - T |
1262 |
| 15.3 | Deviation of the database from the spline fit of thermal conductivity expressed in percent............................................................................................................ | 1265 |
| 16.1 | The recommended values for the latent heat of vaporization of liquid 4He as a function of temperature at the saturated vapor pressure........................................ | 1265 |
| 16.2 | Detail of the recommended values latent heat of vaporization about the lambda transition............................................................................................................ | 1265 |
| 16.3 | The fractional deviation of values of the adopted database from the recommended values for the latent heat expressed in percent...................................................... | 1265 |
| 17.1 | The recommended values for the saturated vapor pressure (in Pa) of liquid 4He calculated from the spline................................................................................... | 1267 |
| 17.2 | Log10 of the saturated vapor pressure curve (in Pa) of liquid 4He............................. | 1267 |
| 17.3 | The fractional deviation of values of the saturated vapor pressure of 4He calculated with the spline from those calculated with the ITS-90 equations expressed as percent.............................................................................................................. | 1267 |
| 17.4 | Deviations in temperature ?T corresponding to the deviations in pressure shown in Figure 17.3........................................................................................................ | 1267 |
Introduction
Liquid
helium has been studied intensively for more than three quarters of a
century: indeed one may advance the claim that it is the most studied
pure substance in the history of science. Our group, pursuing certain
theoretical topics in the 1960's, became aware of the unwelcome impact
of faulty data in testing ideas on the theory of superfluidity. We therefore
began a collection of well-documented data which first appeared in the
book by Donnelly1 and a further set of useful tables appeared in the book
by Wilks2. As research progressed, it became evident that the pressure
of liquid helium is just as significant a parameter as temperature, and
it was recognized that it would be decades before enough data would exist
to make a critical compilation of the properties of liquid helium as a
function of pressure and temperature.
A ray of hope emerged when inelastic neutron scattering studies began
to appear, and one gradually learned the true experimental nature of the
dispersion curve for elementary excitations in He II. Early work by Bendt,
Cowan and Yarnell3 showed that the thermodynamic properties of He II along
the vapor pressure line could be extracted from neutron scattering measurements
with reasonable success. Unfortunately, it soon appeared that the parameters
of the spectrum are both temperature-dependent and pressure-dependent.
The temperature dependence was especially troublesome because the standard
formulas of statistical mechanics, and indeed quantum mechanics, have
no direct provision for handling temperature-dependent energy levels.
This problem was studied by Donnelly and Roberts4 who produced an approximate
method for the computation of thermodynamic quantities which can be used
when the energy levels are known from experiment. The methods of Donnelly
and Roberts then were used by Brooks and Donnelly5 to produce their report
"The Calculated Thermodynamic Properties of Superfluid Helium-4"
which evolved through a thesis, a preliminary edition, and finally a publication
over the period 1972-77. Brooks and Donnelly's tables of equilibrium properties
have the advantage that they are thermodynamically self-consistent over
the entire temperature-pressure (T,P)-plane; that is, they obey Maxwell's
relations; they have the disadvantage that they are not useful near the
-transition. Further, they are based on some ad-hoc assumptions such as
the concept of an "effective sharp spectrum" whose validity
is not known except by the test of utility. Nevertheless, those calculated
tables still stand as the most reliable general guide to the equilibrium
properties of He II over the whole (T,P)-plane as shown in the diagram
below.
![]()
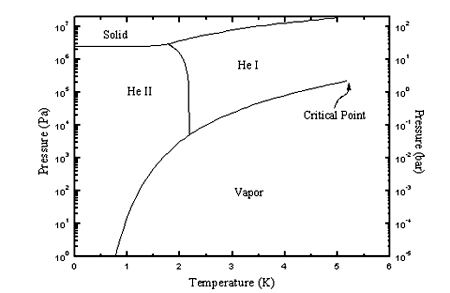
With the issue of this study,
we are presenting a comprehensive collection of the experimental data
for the equilibrium and transport properties of pure liquid 4He.
The format used will generally be the following:
. Chronological
bibliography of all known measurements (placed at the end of each chapter)
2. Selection of an “adopted database” obtained by subjective
judgment. Note that the temperature ranges given in the adopted table
base are in the temperature scale used by the authors, not necessarily
T90.
3. To facilitate the description of the adopted database we have chosen
a table format. The key # is used to give the origin of the data in the
adopted database and leads to the literature citation. To save space we
have used E+2, E+1, E+0, E-1 instead of 102, 10, 1, 10-1 etc.
4. Table of the adopted database. Temperature values in the adopted database
are in T90 Note that the ITS-90 is not defined below 0.65 K. Values below
0.65 K are on the thermodynamic scale.
5. Table of recommended values obtained by the cubic spline fits of the
data.
6. Curves showing the recommended values and the fractional deviation
of values of the adopted database from the recommended values.
If f(T) is the quantity being discussed we define the fractional deviation
in percent as
![]()
![]() =(f
measured -f calculated)/f measured X
100
=(f
measured -f calculated)/f measured X
100
7.
Tables of recommended values.
In most cases we have represented the data by cubic splines. A routine
to evaluate these splines is included as an Appendix.
A word is required about significant figures in this study. Most (but
certainly not all) of the data here is of order one percent in accuracy
so that three figures should be adequate to represent it. Furthermore,
the spline fit returns all the figures yielded by the calculation. Nevertheless
we have usually given recommended data to four figures simply as a compromise
among data of varying precision and accuracy.
A second problem is that some data such as specific heat spans an enormous
range: over 5 decades in (see the recommended values in Chapter 7).
Temperature resolution is another problem. Modern thermometry resolves
temperatures throughout the region to 4K with millikelvin to microkelvin
precision. However the absolute temperature is not known to anything like
this resolution. Most superfluidity researchers have used the T58 vapor
pressure scale and their data is keyed to it for better or for worse.
However the T90 scale is now used to calibrate thermometers, and we have
undertaken the somewhat risky task of converting our T58 tables, which
were circulated as a Report of the Department of Physics, to T90. The
method of doing this was outlined in an MS thesis by Ling Lui in 1992
6.
In some cases we have recalculated temperatures ourselves after discussion
with the authors. Thus a few figures appear which are not exactly those
originally published. Naturally, the biggest changes in data, and greatest
sensitivity to the T90 occurs near the lambda transition.
An even more bizarre situation appears in representing data near the lambda
transition. Here the difference in temperature from the lambda transition
is what is really measured, and these days, that can be done in some cases
to nanokelvin resolution, especially above 2.17K. Thus in the last 100
millikelvin below and above the lambda point we have had to carry many
significant figures because our spline fits the temperature. It is the
joy of least squares cubic splines that they can represent data near a
singular point like the lambda point. The down side is that we are printing
data on temperature with many figures which were really measured as deviations.
The reader will appreciate that any strict treatment of printing only
significant figures will lead to a typographic nightmare.
We can summarize the situation by stating that we are usually erring on
the side of carrying too many figures, and that the antidote is a quick
glance at the normalized deviation curve.
In making the spline fits, we have tried to get as good a fit to the data
as possible, especially near the lambda point. But these fits should under
no circumstances be relied upon for scaling investigations.
Note that we have listed a number of quantities in the units normally
used by the low temperature physics community. Conversion to SI units
has been made in several cases in this report and conversion factors to
SI are given wherever needed.
Our initial work was done using HP BASIC. We list spline evaluation programs
in the Appendix in HP BASIC. In addition, FORTRAN, C, and HP48SX subroutines
are given.
References
1.
Donnelly, R. J.; P.E. Parks and W.I. Glaberson. "Experimental Superfluidity."
University of Chicago Press, Chicago (1967)
2. Wilks, J. "Liquid and Solid Helium." Clarendon Press, Oxford
(1967)
3. Bendt,P.J., R.D. Cowan, J.L. Yarnell. "Excitations in Liquid Helium:
Thermodynamic Calculations." Phys. Rev. 113, 1386-1395 (1959)
4. Donnelly, R.J. and P.H. Roberts. J. Low Temp. Phys. 27, 687-736 (1977)
5. Brooks, J.S. and R.J. Donnelly, J. Phys. Chem. Ref. Data 6, 51-104
(1977)
6. Lui, Ling, “The Observed Properties of Liquid Helium on the ITS-90.”
MS Thesis, University of Oregon (unpublished).
Acknowledgements
These
tables in their present form have been in preparation for some 30 years.
Many students and visitors have had a part in preparing them. From my
own group there have been (in addition to my co-author) James Brooks,
whose thesis made all this possible, Thomas Wagner, James Donnelly, Christa
Laursen, Ling Lui, James Mulder, Robert Riegelmann and most recently,
Steve Stalp, Steve Hall and Rose Lowe-Webb.
Professor Paul Roberts contributed by working out the theory of extracting
thermodynamic properties when energy levels determined by neutron scattering
are temperature dependent, and Dr. R. N. Hills introduced us to the advantages
of cubic splines and wrote the first programs to generate and evaluate
splines, which we still use today. Ron also helped develop the present
tables format.
Ling Lui began the enormous task of converting these tables to T90 as
her master’s thesis. This thesis explains the technical details
of her method of converting to T90.In doing so we had to consult a number
of experts on temperature scale. These were Marty Durieux, Ralph Hudson,
Michael Moldover, Richard Rusby and Clayton Swenson.
When preliminary issues of these tables have been circulated, we have
received many suggestions and new measurements. These have come from Guenter
Ahlers, Dennis Greywall, Horst Meyer, Jay Maynard and Isadore Rudnick.
This research was supported by the National Institute of Standards and
Technology, Office of Standard Reference Data, under grant number NBS788,
by the National Science Foundation through the years under a number of
grants entitled “The Physics of Fluids” and currently, by
the National Science Foundation under grant DMR-9529609.
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |

